Advertisment
Tandem Diabetes Care’s T;SLIM x2 insulin pump is the first automated insulin delivery system to integrate with Abbott’s new FreeStyle Libre 2 Plus sensor
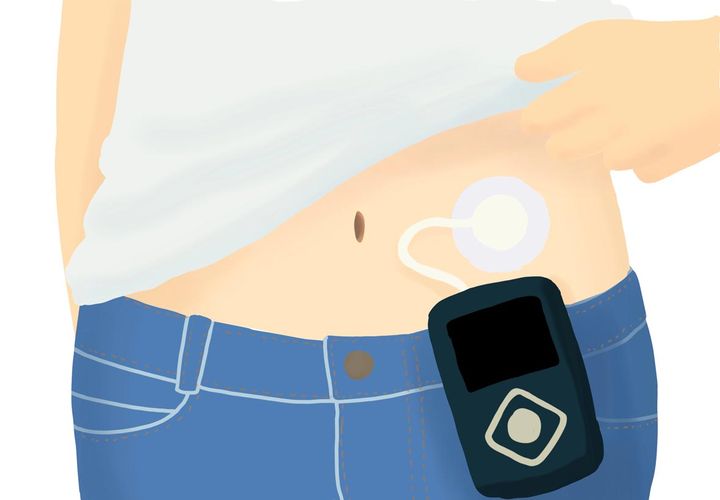
Abbott and Tandem Diabetes Care, Inc. a leading insulin delivery and diabetes technology company, announced that the t:slim X2 insulin pump with Control-IQ technology is the first automated insulin delivery (AID) system to integrate with the newly available FreeStyle Libre 2 Plus sensor, Abbott’s latest continuous glucose monitoring (CGM) technology.
For the first time, FreeStyle Libre technology users in the United States are now able to experience the therapeutic benefits of a hybrid closed-loop system that helps predict and prevent high and low blood sugar.
Tandem’s t:slim X2 insulin pump connects wirelessly to the FreeStyle Libre 2 Plus sensor, which sends automatic glucose readings every minute to the pump. Users can conveniently see their minute-to-minute glucose data on the pump and accompanying t:connect mobile app . The pump’s Control-IQ technology predicts glucose levels 30 minutes into the future, automatically adjusting insulin delivery every five minutes based on CGM readings and can deliver automatic correction boluses (up to one per hour) to help prevent hyperglycemia. When left untreated, hyperglycemia can lead to long-term diabetes complications, such as nerve and kidney damage or disease of the eyes.
‘Tandem’s leadership in AID innovation is underscored with this milestone of launching the first insulin pump to be compatible with Abbott’s CGM technology in the U.S.,’ said John Sheridan, president and chief executive officer of Tandem Diabetes Care. ‘This represents another step forward in our commitment to provide customizable solutions to help reduce burden and create new possibilities for people living with diabetes.’
Abbott’s latest addition to the FreeStyle Libre portfolio is the FreeStyle Libre 2 Plus sensor, which is the modified FreeStyle Libre 2 sensor cleared in 2023 by the FDA for use with AID systems. It is the first and only CGM available in the United States with a wear time of 15 days for both adults and children. Demonstrating proven accuracy in overall glucose readings with an 8.2% MARD, the sensor is small, discreet and comfortable to wear.
Last year, Abbott received clearance from the FDA for a modified version of its Freestyle Libre 2 sensor that can be used with automated insulin delivery systems.





