New SPECTREM study findings reveal Tremfya (guselkumab) effectively clears overlooked and undertreated plaque psoriasis – Johnson & Johnson
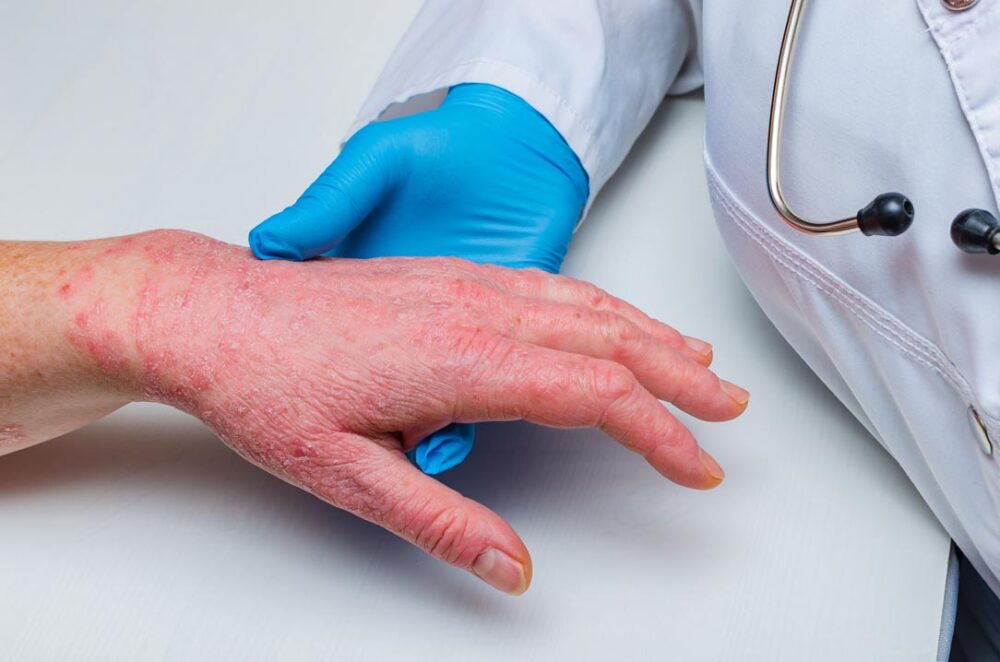
Johnson & Johnson announced that treatment with Tremfya (guselkumab) resulted in clear or almost clear skin in the majority of adults with low body surface area (BSA) moderate plaque psoriasis (PsO) with special site involvement who had failed topical treatment. Sensitive or highly visible areas affected by PsO, including the scalp, face, skin folds and genitals, are considered “special sites” and can have significant impact on patients’ daily lives, yet systemic treatment is infrequently provided and this group of patients remains largely undertreated. Data from the Phase IIIb SPECTREM study, the first prospective, large-scale, randomized-controlled, double-blind clinical study to measure skin clearance and other treatment outcomes in low BSA moderate PsO with involvement across four special sites (scalp, face, skin folds and genitals) and previous topical treatment failure, were presented today at the 2024 Fall Clinical Dermatology Conference.
“People who have special site plaque psoriasis with lesions that cover a smaller total area of their body are often only prescribed topical treatments and not considered candidates for advanced therapies, as treatment decisions are often driven by body surface area coverage and not symptomatic burden,” said Linda Stein Gold, MD, Director of Dermatology Clinical Research at Henry Ford Health, and SPECTREM investigator. “Results of the SPECTREM study could represent a new approach to care for patients with low body surface area psoriasis, as the majority of patients treated with Tremfya achieved clear or almost clear skin.”
SPECTREM Week 16 Results Show High Levels of Skin Clearance; A significantly greater proportion of patients who received Tremfya achieved the primary endpoint of an Investigator’s Global Assessment (IGA) score of cleared (0) or minimal disease (1) compared to those who received placebo (74.2% versus 12.4%, respectively; p<0.001). These results were comparable irrespective of baseline BSA. i. Results Demonstrate High Levels of Skin Clearance in Patients with Special Site Involvement; a. Significant clearance (defined as site-specific IGA 0/1 response) versus placebo at Week 16: scalp (75.0% versus 14.5%), face (87.8% versus 28.6%), intertriginous (86.5% versus 28.8%) and genital (78.0% versus 37.5%) (all comparisons p<0.001). b. Complete clearance of each special site was consistently achieved in the majority of patients who received Tremfya versus placebo: scalp (60.3% versus 9.3%), face (75.7% versus 23.9%), intertriginous (76.6% versus 24.2%) and genital (72.7% versus 32.7%).
ii. Statistically significant improvements were also achieved across all major secondary endpoints, including: a. 52.9% achieved a Psoriasis Area Severity Index (PASI) 90 response compared to 6.2% of participants who received placebo (p<0.001). b. The average patient saw over 80% improvement from baseline for both BSA and PASI compared to .those who received placebo (80.6% versus 6.1% and 82.6% versus 13.7%, respectively; p<0.001).
iii. Participants Report Improved Quality of Life Impact After Three Doses of Tremfya; a. A greater proportion of Tremfya-treated patients achieved: Dermatology Life Quality Index (DLQI) scores of 0/1 compared to patients receiving placebo (48.9% versus 3.5%). A 4-point greater improvement from baseline in Psoriasis Symptoms and Signs Diary (PSSD) itch compared to placebo (62.7% versus 12.5%).
The overall safety in the SPECTREM study was consistent with the established Tremfya safety profile.
About SPECTREM (NCT06039189)1SPECTREM is a Phase IIIb, multicenter, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of Tremfya (guselkumab) versus placebo for the treatment of low body surface area (BSA) moderate plaque PsO with special site involvement. Patients (n=338) were randomized approximately 2:1 to receive Tremfya 100 mg subcutaneous injection at Weeks 0 and 4, then every eight weeks (q8w) or placebo at Weeks 0 and 4, followed by crossover to Tremfya at Week 16 and q8w. The study was designed to evaluate the efficacy and safety of Tremfya in patients with a diagnosis of PsO, BSA 2-15% with at least one plaque outside of special sites, per Investigator’s Global Assessment (IGA) 3, and involvement of at least one qualifying special site, including scalp, face, intertriginous, or genital, with at least moderate severity. The primary endpoint of the study is the percentage of patients who achieve an IGA score of cleared (0) or minimal (1) at Week 16; overall lesions are graded for induration, erythema, and scaling. The SPECTREM study is ongoing with an active treatment period from Weeks 16-48 to evaluate final efficacy and from Weeks 16-56 to evaluate final safety.
Through Week 16, 37.8% of patients who received Tremfya experienced at least one adverse event (AE), compared to 39.8% who received placebo. Three patients who received Tremfya experienced a serious AE, compared to 1 patient who received placebo. There were no discontinuations due to an AE in patients who received Tremfya, while 4 patients who received placebo discontinued because of an AE.
The study is evaluating approximately 338 patients from the U.S. and Canada, who will be treated and followed for 56 weeks. SPECTREM enrolled nearly the same number of male and female participants in both the Tremfya and placebo treatment groups (51.6% and 48.4% vs. 50.4% and 49.6%), which reflects the similar prevalence of the disease in both men and women.
About VISIBLE (NCT05272150); VISIBLE is a Phase IIIb, multicenter, randomized, double-blind, placebo-controlled (Weeks 0-16) trial in adult patients (≥18 years of age) with moderate to severe body and/or scalp PsO. Patients (n=211) were randomized to Tremfya100 mg subcutaneous injection at Weeks 0, 4, and 12, then q8w; placebo at Weeks 0, 4, and 12, followed by crossover to Tremfya at Week 16, Week 20, and q8w. The study was designed to evaluate the efficacy and safety of Tremfya in skin of color patients (self-identify as non-white) across the entire spectrum of the Fitzpatrick scale (I-VI). The study consisted of 2 cohorts, Cohort A: moderate to severe plaque PsO (IGA ≥3, PASI ≥12, and BSA involvement of ≥10%) and Cohort B: moderate to severe scalp PsO (SSA ≥30%, PSSI ≥12, ss-IGA ≥3, and ≥1 plaque outside the scalp) for at least 6 months before study administration, or central photo review expert confirmed PsO diagnosis, or biopyasy confirmed PsO. The VISIBLE study is still ongoing with an active treatment period from Weeks 16-48 and long-term extension through Week 112 where patients continued receiving Tremfya q8w.
The study will evaluate approximately 211 participants from the U.S. and Canada who will be treated and followed for approximately two years.





