Advertisment
European Commission approval for Cosentyx as first and only IL-17A inhibitor for hidradenitis suppurativa – Novartis
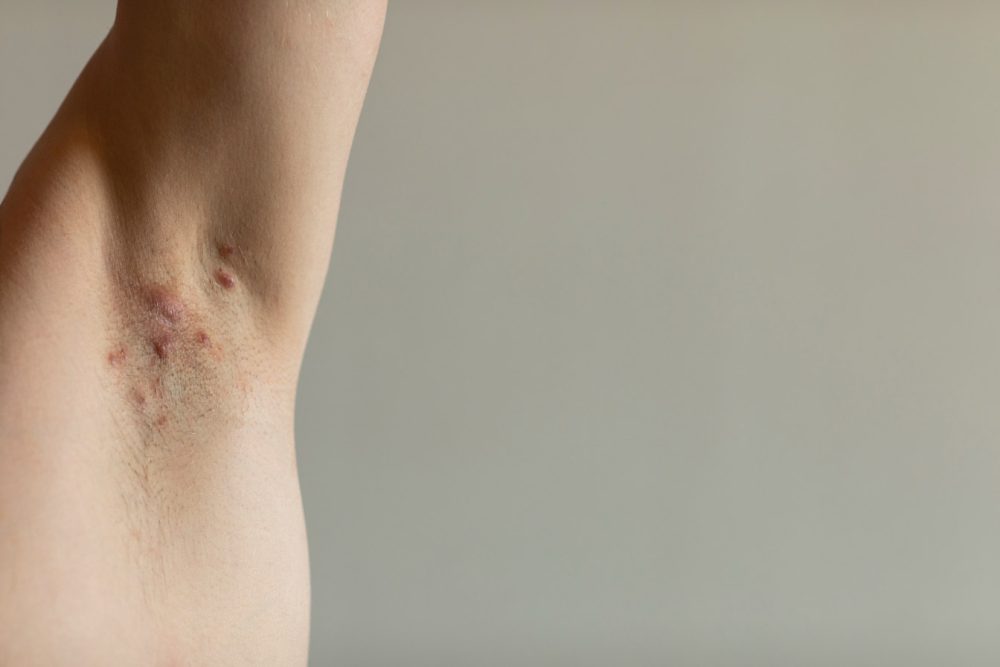
Novartis announced that the European Commission (EC) has approved Cosentyx (secukinumab) for use in adults with active moderate to severe hidradenitis suppurativa (HS) and an inadequate response to conventional systemic HS therapy.
“With only one currently approved treatment option, I see HS patients with a tremendous need for alternatives that reduce the disabling physical symptoms of HS, improve the emotional burden and help partially avoid invasive surgery, if treating early,” said Professor Christos C. Zouboulis, President of the European Hidradenitis Suppurativa Foundation, Director of the Departments of Dermatology, Venereology, Allergology and Immunology, Städtisches Klinikum Dessau, and Founding Professor of Dermatology and Venereology at the Brandenburg Medical School, Germany. “This expanded approval offers physicians an additional effective and, for dermatologists, familiar treatment choice that we can feel confident in prescribing for this complex and challenging disease.”
HS is a chronic inflammatory skin disease resulting in painful and potentially disfiguring abscesses; around 200,000 people in Europe currently live with moderate to severe stages of the condition.





