Advertisment
National approvals in all EU countries for MOB 15 to treat mild to moderate fungal infections of the nails – Moberg Pharma
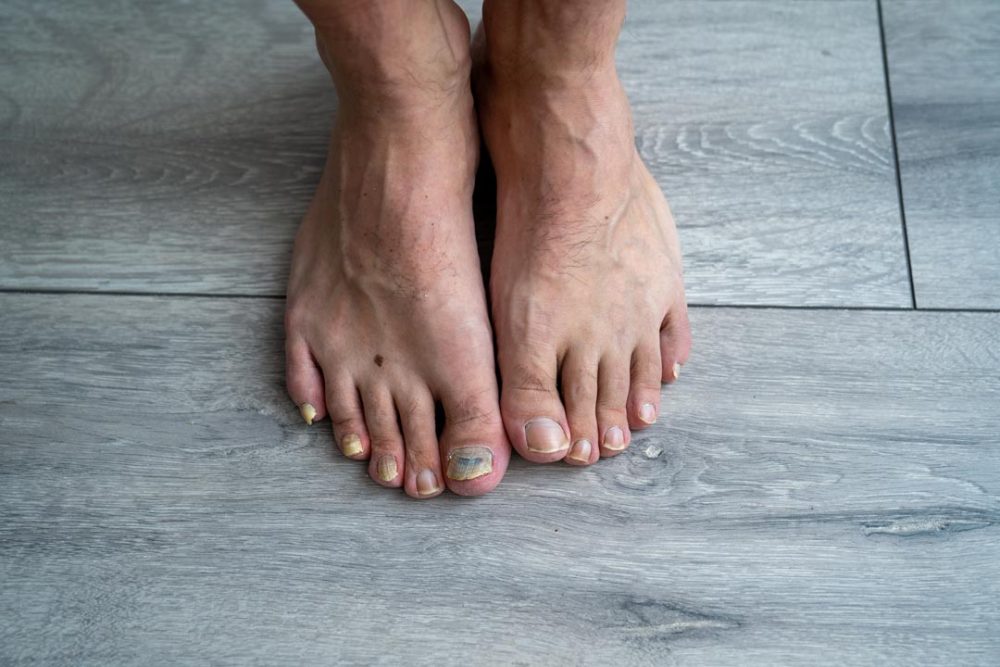
Moberg Pharma AB hereby announces that MOB 015 has received national approvals for all countries included in the decentralized procedure. MOB 015 is thus approved for the treatment of mild to moderate fungal infections of the nails in adults in 13 European countries.
National approvals follow the completion of the decentralized procedure with a positive result where MOB 015 is recommended for approval in 13 European countries, see press release from June 28th, 2023. The following EU countries are included: Austria, Belgium, Czech Republic, Denmark, Finland, France, Hungary, Ireland, Italy, Netherlands, Norway, Spain and Sweden.
MOB -015 can be obtained with a prescription (Rx) in the Czech Republic, Denmark, Finland, France, Ireland and Spain, while it’s approved as a non-prescription medicinal product, i.e. over-the-counter (OTC) in Austria, Belgium, Hungary, Italy, the Netherlands, Norway and Sweden.





