Advertisment
Journal of the American Academy of Dermatology publishes Zorvye (roflumilast) foam, 0.3% results for seborrheic dermatitis from pivotal phase III trial – Arcutis Biotherapeutics Inc
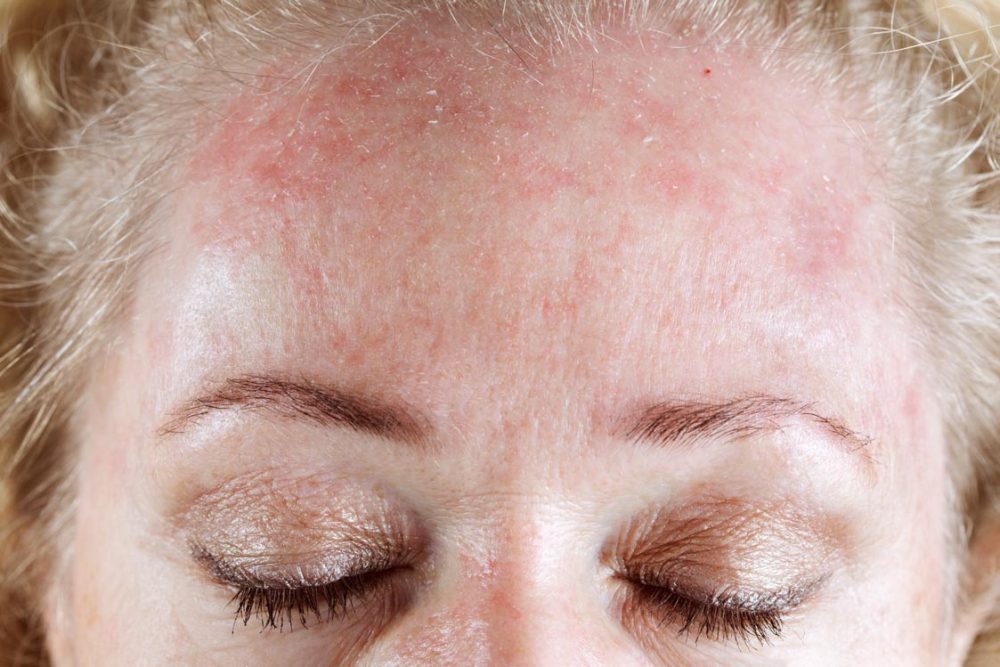
Arcutis Biotherapeutics, Inc. announced that the Journal of American Academy of Dermatology (JAAD) published positive results from the pivotal Phase III STRATUM trial evaluating Zoryve (roflumilast) foam, 0.3% as a once-daily steroid-free treatment for seborrheic dermatitis.
The article was published online, and found that treatment with Zoryve foam was superior to vehicle, with 80% of individuals achieving the primary efficacy endpoint of Investigator Global Assessment (IGA) Success and 51% of individuals reaching complete clearance at Week 8. Zoryve foam was approved by the FDA for treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older in December 2023 and is the first drug approved for seborrheic dermatitis with a new mechanism of action in over two decades.
“Despite being very common, seborrheic dermatitis has traditionally been a disease with limited treatment options. It also can have a significant impact on quality of life,” stated Dr. Andrew Blauvelt, MD, MBA, lead study author and investigator at the Oregon Medical Research Center. “The publication of the Phase III STRATUM study results in the Journal of American Academy of Dermatology further validates the significance of roflumilast foam as a new treatment option for seb derm, one that provides treatment success in eight of ten patients, along with significant and rapid improvements in key signs and symptoms of disease, as early as two weeks. These results highlight the effectiveness and safety of roflumilast foam, a steroid-free treatment and the first novel mechanism of action approved for seb derm in two decades. It should end suffering from this long-neglected condition.”
See- “Roflumilast foam 0.3% for adolescent and adult patients with seborrheic dermatitis: A randomized, double-blinded, vehicle-controlled, phase III trial”,: Journal of the American Academy of Dermatology. Pre-Proof Published online: January 20, 2024 Andrew Blauvelt, Zoe D. Draelos, Linda Stein Gold, Javier Alonso-Llamazares, Neal Bhatia,Janet DuBoisa and others.





