Advertisment
Phase III CARISEL study of Vocabria + Rekambys shows long-acting regimen is achievable in HIV – ViiV Healthcare
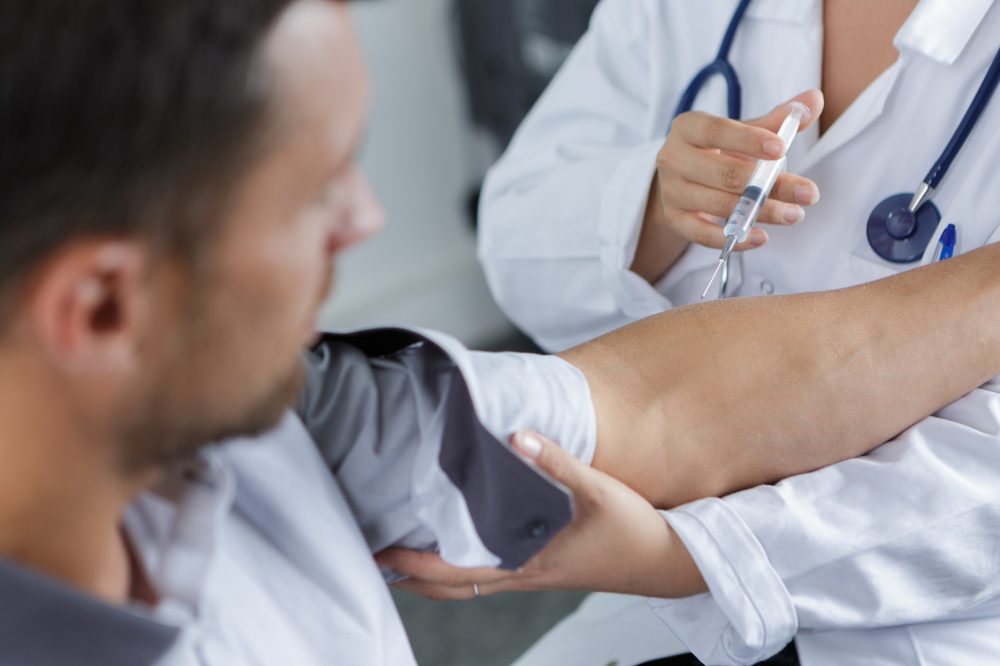
ViiV Healthcare presented positive interim data from the Phase III CARISEL study of Vocabria + Rekambys (cabotegravir + rilpivirine), which was initiated and conducted during the COVID-19 pandemic. The study evaluated perspectives of healthcare teams and people living with HIV, through surveys and interviews, around the implementation of Vocabria (cabotegravir injection) and Janssen Pharmaceutical Companies of Johnson & Johnson’s Rekambys (rilpivirine long-acting injectable suspension ) administered every 2-months, with data showing that implementation of the long-acting regimen is realistic and achievable in a variety of European healthcare settings.
Implementation concerns that were identified among healthcare teams at the start of the study reduced markedly when compared to the baseline across all European countries involved once the study began. The barriers cited as being the most “moderately” to “extremely concerning” at Month 1 included risk of resistance (36%, compared to 16% at Month 5), enough staffing (34%, compared to 18% at Month 5), and injection pain/soreness (34%, compared to 25% at Month 5).
Additional findings from the interim CARISEL data include the facts that most people living with HIV who started treatment continued to feel positive about the long-acting regimen, with 91% feeling positive at Month 4 vs 84% at Month 1. Healthcare teams also felt positive about the long-acting regimen, with 81% feeling positive at Month 5. Most people living with HIV on the trial agreed or completely agreed that long-acting cabotegravir and rilpivirine was highly acceptable, appropriate and feasible to implement (mean scores of 4.6, 4.6, 4.6 respectively on a 5-point Likert scale).
Most healthcare teams found long-acting cabotegravir and rilpivirine acceptable for people living with HIV citing factors including eliminating worry about carrying and taking HIV pills (44%). Interim findings were presented at the 18th European AIDS Conference (EACS 2021) being held 27-30 October.





