Advertisment
Keytruda monotherapy is EU approved for adult and pediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma – Merck Inc
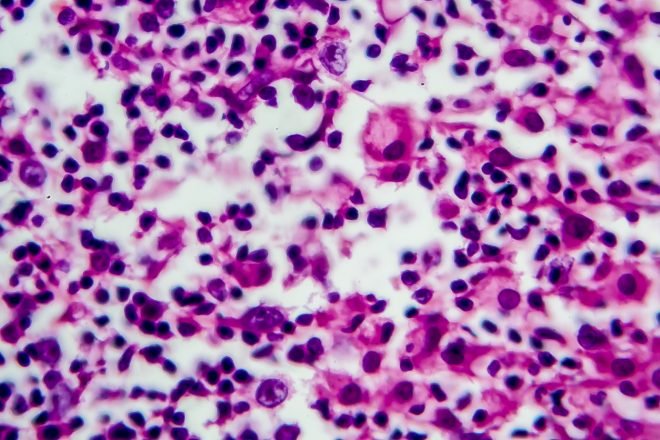
Merck announced that the European Commission (EC) has approved an expanded label for Keytruda, Merck’s anti-PD-1 therapy, as monotherapy for the treatment of adult and pediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option.
This approval is based on results from the pivotal Phase III KEYNOTE-204 trial, in which Keytruda monotherapy demonstrated a significant improvement in progression-free survival (PFS) compared with brentuximab vedotin (BV), a commonly used treatment. Keytruda reduced the risk of disease progression or death by 35% (HR=0.65 [95% CI, 0.48-0.88]; p=0.0027) and showed a median PFS of 13.2 months versus 8.3 months for patients treated with BV. The approval is also based on supportive data from an updated analysis of the KEYNOTE-087 trial; KEYNOTE-087 was the basis for the 2017 EC approval of Keytruda for the treatment of adult patients with relapsed or refractory cHL who have failed ASCT and BV or who are transplant ineligible and have failed BV. This approval is the first pediatric approval for Keytruda in the European Union (EU).





