Advertisment
Investigative bimekizumab shows significant efficacy in plaque psoriasis
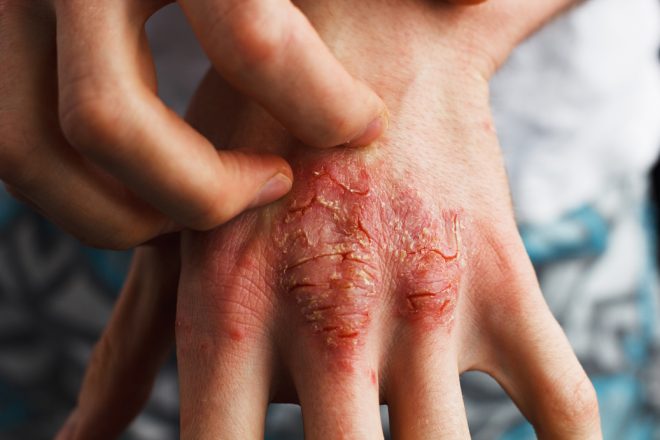
Article written by Bruce Sylvester
Researchers report that patients treated with bimekizumab, a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A, have achieved significant improvements in moderate to severe plaque psoriasis.
The findings appeared on Feb. 6, 2021 in The Lancet.
“Bimekizumab showed high levels of response, which were durable over 56 weeks, with both maintenance dosing schedules (every 4 weeks and every 8 weeks),” the authors said.
The BE READY study was a phase 3, randomized, double-blind, placebo-controlled trial conducted internationally at 77 sites.
The investigators stratified by region and previous biologic exposure subjects aged 18 years or older diagnosed with moderate to severe plaque psoriasis. They randomized the subjects (4:1) to receive bimekizumab 320 mg every 4 weeks or placebo every 4 weeks.
The two primary endpoints were the proportion of subjects achieving 90% or greater improvement from baseline in the Psoriasis Area Severity Index (PASI90), and the proportion of subjects achieving a score of 0 (clear) or 1 (almost clear) on the five-point Investigator’s Global Assessment (IGA) scale at week 16.
Subjects treated with bimekizumab who achieved PASI90 at week 16 were re-assigned (1:1:1) to bimekizumab 320 mg every 4 weeks, every 8 weeks, or placebo for weeks 16–56.
The researchers reported that at week 16, 317 (91%) of 349 subjects treated with bimekizumab 320 mg every 4 weeks achieved PASI90, compared with one (1%) of 86 patients receiving placebo, a significant difference. (p<0·0001)
Also, 323 (93%) of 349 subjects receiving bimekizumab 320 mg every 4 weeks achieved an IGA score of 0 or 1 versus one (1%) of 86 subjects receiving placebo, a significant difference (p<0·0001).
Notably, these responses continued out to week 56 among subjects treated with bimekizumab 320 mg every 8 weeks and every 4 weeks.
The researchers reported treatment-emergent adverse events out to week 16 in 213 (61%) of 349 subjects receiving bimekizumab 320 mg every 4 weeks and 35 (41%) of 86 subjects receiving placebo every 4 weeks.
Between weeks 16 and 56, treatment-emergent adverse events appeared in 78 (74%) of 106 subjects receiving bimekizumab 320 mg every 4 weeks, 77 (77%) of 100 subjects receiving bimekizumab 320 mg every 8 weeks, and 72 (69%) of 105 subjects receiving placebo.
None of the safety findings were a surprise. “Moreover, bimekizumab was well tolerated, with no unexpected safety findings,” the authors said.
“Data presented here further support the therapeutic value of bimekizumab and inhibition of IL-17F in addition to IL-17A for patients with moderate to severe plaque psoriasis,” the authors concluded.





