Advertisment
Novaliq + Laboratoires Théa announce partnership and EU approval for Vevizye for dry eye disease
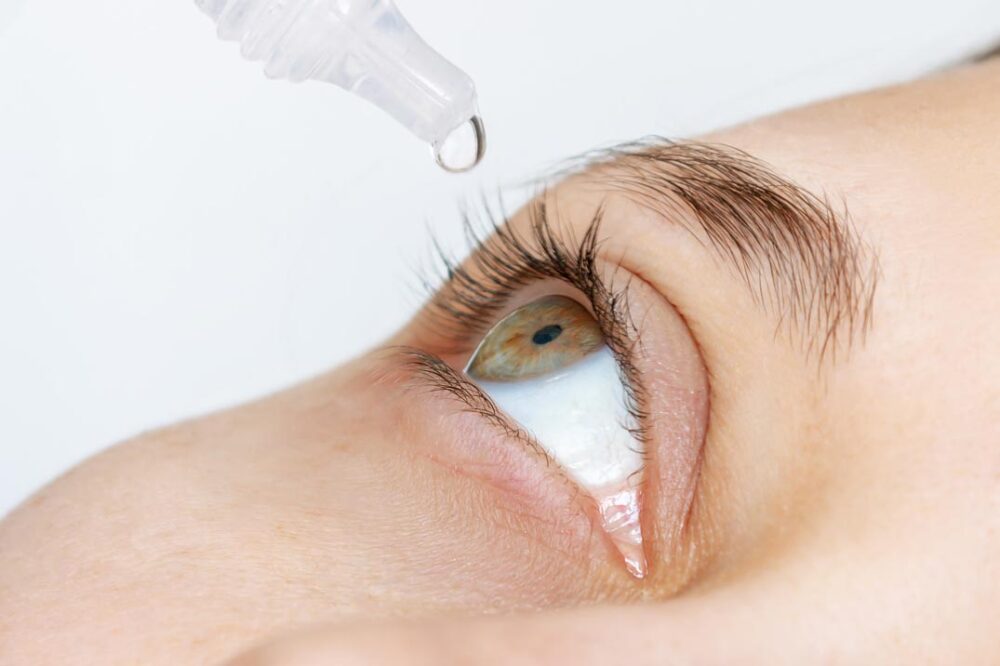
Novaliq, a biopharmaceutical company focusing on first- and best-in-class ocular therapeutics based on the unique EyeSol water-free technology, and Laboratoires Théa (Théa), the leading independent eye care group in Europe, announce European Commission approval of Vevizye (ciclosporin 0.1% eye drops solution) in Europe and the closing of a partnership under which Théa has acquired the rights to commercialise the product in Europe and selected countries in the Middle East and North Africa (MENA).
Vevizye is based on Novaliq’s proprietary EyeSol technology and the only water-free ciclosporin 0.1% eye drops solution approved in the European Union for the treatment of moderate to severe dry eye disease (keratoconjunctivitis sicca) in adult patients which has not improved despite treatment with tear substitutes.
Dry eye disease (DED) is one of the most common ocular surface disorders, affecting approximately 15 million diagnosed patients in the five largest European countries and is difficult to treat. In Europe, there is a need for additional prescription treatments intended for adults suffering from moderate to severe dry eye disease.
About ESSENCE 1 & ESSENCE 2 clinical studies; The efficacy of Vevizye for the treatment of dry eye disease was demonstrated by two randomised, multicentre, double-masked, vehicle-controlled studies (ESSENCE-1) and ESSENCE-2)..The change from baseline in total corneal fluorescein staining (tCFS) score at Day 29 was the primary endpoint in both trials. At Day 29, a statistically significant reduction in tCFS favouring Vevizye was observed in both studies. Up to 71.6% of patients responded within four weeks with a clinically improvement in total corneal fluorescein staining. All other key secondary ocular surface sign endpoints (tCFS at Day 15, conjunctival staining at Day 29 and central corneal staining at Day 29) showed statistically significant effects favouring Vevizye in both studies. Continued improvement under therapy in both signs and symptoms of DED were demonstrated over a period of up to 56 weeks.





