Advertisment
Could pneumatic tube delivery systems damage protein-based drugs?

29th EAHP Congress highlights
Biopharmaceuticals are complex biological molecules that require careful storage and handling to ensure medication integrity. They are especially sensitive to mechanical stress and shaking, temperature excursions and light exposure.1 A major concern is that conditions during transport of compounded drugs could lead to aggregation of proteins and consequent changes in immunogenicity. Thus, a high-quality, compounded product could be prepared in a centralised pharmacy facility but could then be altered during transport to wards or clinics. As some hospitals use pneumatic tube delivery systems for rapid transport of medicines and other items between wards and departments, this is an issue of concern.
Kjellstrom and colleagues2 described a project to explore the impact of hospital transport systems, including pneumatic tubes, on protein stability in iv bags and syringes. They noted that the use of pneumatic tubes is prohibited in many hospitals after studies demonstrated increased numbers of subvisible particles from protein aggregates.
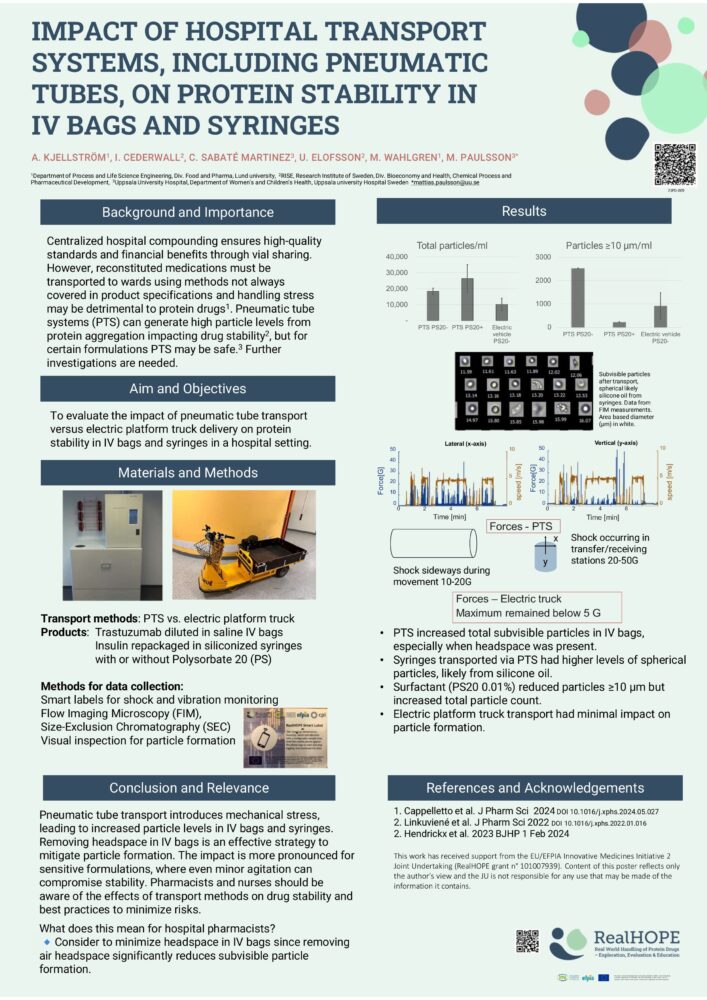
This study examined the impact of pneumatic tube systems and electric platform trucks on protein stability in IV bags and ready-to-use syringes. Smartlabels (CPI, UK) were used for shock and vibration monitoring. Flow imaging microscopy, size exclusion chromatography and visual inspection measurements were performed.
The results showed that transport via pneumatic tubes was associated with:
- increased levels of spherical particles in syringes – thought to be most likely silicone oil
- increased numbers of sub-visible particles in IV bags with headspace (with and without added drugs)
Furthermore, the addition of polysorbate 20 prevented particles over 10 micrometers, but increased total numbers of particles.
The authors concluded that high shock levels during transport using pneumatic tubes appeared to release particles from the material of both syringes and IV bags. They recommended removing headspace during transport of IV bags, especially if using pneumatic tube systems.
References
- Sabaté-Martínez C, Paulsson M, González-Suárez S, Elofsson U, Fureby AM, Wahlgren M, López-Cabezas C. How are we handling protein drugs in hospitals? A human factors and systems engineering approach to compare two hospitals and suggest a best practice. Int J Qual Health Care. 2024 Mar 18;36(1):mzae020. doi: 10.1093/intqhc/mzae020. PMID: 38462489; PMCID: PMC11002458.
- Kjellström A, Cederwall I, Sabaté Martinez C, Elofsson U, Wahlgren M, Paulsson M. Impact of hospital transport systems including pneumatic tubes, on protein stability in iv bags and syringes. (Poster) EAHP Congress 2025