Advertisment
Monoclonal antibodies for migraine: real-world findings

28th EAHP Congress Highlights
No fewer than 11 posters from Spanish pharmacists dealt with the use of monoclonal antibodies in the management of migraine. Anti-CGRP (calcitonin gene-related peptide) antibodies bind to the receptor (e.g. erenumab) or to the CGRP molecule itself (e.g. eptinezumab, fremanezumab, and galcanezumab). Aspects explored by the Spanish researchers included real-world safety and effectiveness, switching between products and quality of life.
Real-world safety and effectiveness
Garcia-Lastra and colleagues (Aviles, Spain; poster 5PSQ-053) conducted a retrospective study of 32 patients who had received galcanezumab for prevention of migraine. Treatment was considered effective if there was a reduction of at least 50% in the migraine days per month (MDM) or a reduction of more than five points on the Headache Impact Test (HIT-6) score. The median duration of treatment was 19 months. The authors reported that the MDM fell from 15 to 5 and the HIT-6 score fell from 69 to 57. At the end of the study period on 15.4% of patients had discontinued the drug due to side effects or ineffectiveness.
Estrada and colleagues (Badalona, Spain; poster 4CPS-043) assessed the one-year safety and efficacy of prophylactic anti-CGRP treatment with erenumab, galcanezumab and fremanezumab. A total of 42 patients was included. The authors noted that high response rates (more than 50% reduction in monthly migraine days (MMD)) were achieved with all three agents. Only three discontinued treatments due to adverse effects.
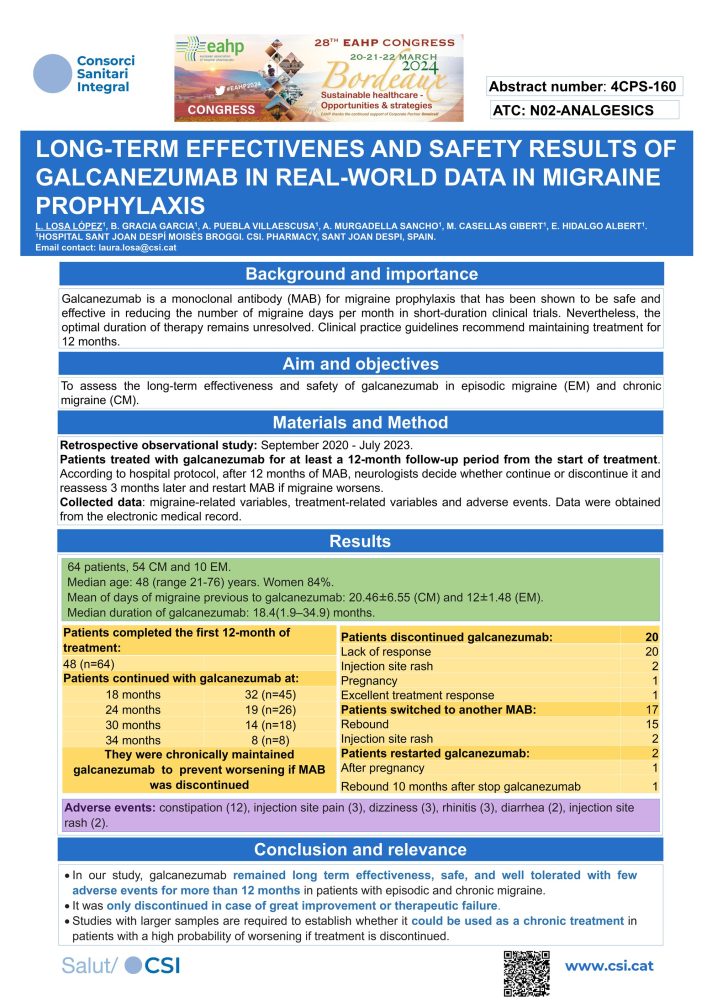
Losa-Lopez and colleagues (Sant Joan Despi, Spain; poster 4 CPS-160) examined the outcomes of long-term (12 months or more) prophylactic treatment of 64 people with chronic and episodic migraine. The mean number of migraine days (before galcanezumab treatment) was 20.46 ± 6.55 days per month for chronic migraine and 12±1.48 for episodic migraine and the mean duration of treatment was 18.4 (1.9 – 34.9) months. It is noteworthy that 20 patients discontinued galcanezumab because of lack of response.
Diaz-Perales and colleagues (Malaga, Spain; poster 5PSQ-072) looked at the real-world safety of galcanezumab in patients with chronic migraine. They reported that 17 of 110 patients discontinued treatment and of these, 29.4% did so because of intolerance. Reactions included weight gain, constipation generalised itching and local reactions. The authors noted that discontinuation due to drug intolerance was lower than reported in the pivotal trials.
Ojeda and colleagues (Barcelona, Spain; poster 4CPS-091) examined real-life persistence with treatment in 207 people who received prophylactic fremanezumab. At three-month and six-month follow-ups, persistence was 92.6% and 80.0%, respectively. The percentages of people who achieved a 50% reduction in monthly migraine days was 56.8% at three months and 54.5% at six months. A total of 27% of people developed side effects, the most common of which were allergic reactions or pruritus.
Therapeutic breaks
Some local guidelines advise a therapeutic break after 12 months treatment with an anti-CGRP agent. Two groups focused on this aspect of anti-CGRP use.
De Castro-Avedillo and colleagues (Leon, Spain; poster 4CPS-144) described the patterns of drug usage in 74 patients treated with erenumab or galcanezumab. They reported that about half the patients had to restart treatment after a therapeutic break of about six months. Of these, about half restarted with the same drug.
Similarly, Gomez and colleagues (Santander, Spain; poster 4CPS-020) described the patterns of drug usage with erenumab and galcanezumab in 273 patients. They concluded that their results did not demonstrate high effectiveness after 12 months. Moreover, there was no difference between the two drugs.
Switching between monoclonal antibodies
In clinical practice switching between different anti-CGRP monoclonal antibodies occurs but there are no clinical trial data to support the effectiveness of this practice.
Cantudo-Cuenca and colleagues (Granada, Spain; poster 4CPS-018) analysed results from 215 patients who had failed to respond to treatment with a monoclonal antibody for three months and had then switched to another class of monoclonal antibody. They identified 110 patients treated with galcanezumab or fremanezumab (both CGRP-ligands), 24 of whom were switched to the CGRP receptor antibody, erenumab. Of 105 patients treated with erenumab, 30 were switched to a CGRP-ligand. They noted that this appeared to be a promising approach.
Mayo and colleagues (Madrid, Spain; poster 4CPS-202) reviewed a total of 55 treatments in 18 patients who had received, for at least three months, fremanezumab (225mg/month), galcanezumab (120mg/month) and erenumab (140mg/month). The mean number of headache days at baseline was 20.6 (SD 7.8) days. However, their results showed that only a maximum of 16% of patients could be ‘rescued’, taking a 30% decrease in in the number of headache days per month as the efficacy threshold.
Symptomatic medication overuse
Chronic migraine is associated with the risk of analgesic overuse or abuse. Tudela and colleagues (Cadiz, Spain; poster 4CPS-115) evaluated 54 people suffering from chronic migraine who received at least three months treatment with fremanezumab (225mg monthly injection). The median duration of treatment was 12 months. Forty people were converted to episodic migraineurs with the monthly number of headache days falling from 28.7 (95% CI 27.1 – 30.0) to 4.0 (95% CI 3.9 – 6.4). and episodic migraine. Importantly, the percentage of these people who were classified as ‘symptomatic medication over-users’ decreased from 97.5% to 2.5%.
Quality of life
Velez and colleagues (Leon, Spain; poster 4CPS-010) evaluated quality of life (QoL) using EQ-5D-5L questionnaire in 32 patients receiving erenumab for chronic or episodic, high frequency migraine. The results showed significant reductions in frequency and intensity of migraines for 30 responders and corresponding increases in the quality of life (fewer problems with daily activities, anxiety/depression and mobility). Two patients stopped treatment due to lack of response.
The 28th EAHP Congress took place in Bordeaux, France 20th-22nd March 2024. The congress theme was: Sustainable healthcare – opportunities and strategies.





