Advertisment
Fluid Stewardship – Definitions, Concerns and Opportunities

Why is fluid stewardship needed?
“Fluid stewardship is needed… because fluids are the most common drug administered in an ICU patient or in a hospitalized patient, whether it is intentional or unintentional”, said Dr. Anthony Hawkins (Clinical Associate Professor, University of Georgia College of Pharmacy). It is particularly important to avoid fluid overload which can be associated with acute kidney injury and higher mortality, he explained.
Conventional teaching is that one liter of sodium chloride 0.9% should be given every eight hours for maintenance treatment. However, patients also receive additional fluids in the form of blood (each unit provides 300 mL), flushes (typically a 20 mL flush before and after administration of intravenous medication), medicines administered intravenously and nutrition. This phenomenon is known as ‘fluid creep’ and can account for significant volumes of fluid.1 Ideally, all of these should be taken into account and fluid administration modified accordingly, he said. Fluid creep is also associated with an increasing sodium burden. Just 17 IV flushes deliver 1.5 G salt – “as much sodium as a salt-restricted diet” – and this is often not captured by input/output charts, he added.
The cumulative burden of fluid creep contributes significantly to fluid (and sodium) overload. Fluid overload is defined as an increase in body weight of 10 percent or more. Volume overload has significant effects on organ function – for example, it can cause acute kidney injury (AKI).2 In this situation, a small increase in serum creatinine level or decrease in urine output could trigger prescribing of more fluids and lead to a worsening problem. Studies have clearly shown a relationship between fluid overload and an increase in mortality at 90-days.3-8 Moreover, fluid overloaded patients are less likely to survive or ambulate at discharge and are more likely to spend time on ventilator, develop AKI requiring renal replacement treatment, and be discharged to a secondary care facility instead of home.8-11
Composition of IV fluids
Normal saline (0.9% sodium chloride solution) is far from physiologically normal and this can give rise to a number of problems, Dr. Hawkins explained. It has a similar osmolarity to that of plasma but the pH is lower (5.7 vs 7.4) and the chloride content is considerably higher (154 mmol/L vs 103 mmol/L). The acidity and high chloride content can contribute to the development of hyperchloremic metabolic acidosis. Other solutions use alternative anions that are readily metabolized in the liver e.g. lactate (Ringer’s solution) or acetate (in Multiple Electrolytes Solution), he noted.
As little as one liter of 0.9% sodium chloride solution delays time to make urine compared to balanced crystalloids, according to studies in healthy individuals. A high chloride load is associated with decreased renal blood flow, hyperchloremic metabolic acidosis and acute kidney injury, he emphasized.9, 12-18
“If you are going to give fluid, saline is the wrong answer in most circumstances”, said Dr. Hawkins. In fact, many studies have shown that balanced crystalloid solutions are superior to 0.9% sodium chloride, he said.
Turning to the other ingredients in common, large-volume intravenous fluids, Dr. Hawkins pointed out that both Lactated Ringer’s solution and Multiple Electrolytes Solution contain small amounts of potassium (4 and 5 mEq/L (mmol/L) respectively). In these quantities “the benefits outweigh the problems”, he said, noting that 10 liters would have to be administered rapidly to raise the serum potassium level by 0.4 mEq/L (mmol/L).
Lactated Ringer’s solution contains 28 mEq/L (mmol/L) of lactate but this presents no problem (i.e. risk of lactic acidosis) in the absence of end-stage liver disease. “The healthy liver has enough clinical reserve to convert lactate to bicarbonate”, said Dr. Hawkins. Lactated Ringer’s solution has a relatively low osmolarity (273 mOsm/L) – and this could be a cause for concern if there is traumatic brain injury, a situation where hypertonic fluids are required, he explained.
Fluid optimization
Fluid optimization and personalization is important for the bedside clinician to be able to make a patient-centered decision about whether the patient needs vasopressors, IV fluids or maybe even diuretics. This requires good knowledge of the properties of IV fluids and the ability to assess the patient’s volume status and fluid responsiveness, explained Dr. Hawkins.
Different IV fluids have different impacts on intravascular and extravascular volumes. Some fluids remain in the intravascular compartment whilst others leak into the extravascular compartment. The choice of fluid depends on the clinical goals for the patient at the time. “It’s important to know which type of fluid does what – and to know a little bit about assessing volume status so that you can make a good clinical decision to give or not to give IV fluid”, he said.
Administration of IV fluids to someone who already has pulmonary and peripheral edema will result in further deterioration with worsening of the pulmonary edema. On the other hand, if vasopressors are administered to raise blood pressure in a patient who is “intravascularly dry”, there is a risk that this could cause arterial clampdown and digital ischemia.
Sepsis is a good example of a situation where careful fluid management can be critical.
In summary, “there is a 0.8 -1.0% increase in in-hospital or 30-day mortality when you don’t practice fluid stewardship”, said Dr. Hawkins.19 This can be due to simple volume overload or, as the literature suggests, inappropriate use of 0.9% sodium chloride rather than balanced electrolyte solutions. “When you look at the data to see how much volume it takes – it only takes 1-2 liters of IV fluid to increase a patient’s chance of death by roughly 1%”, he emphasized.16 Clearly there are opportunities for pharmacists to implement fluid stewardship strategies, he added.
Getting started in fluid stewardship
The ROSE model provides a useful framework for fluid stewardship activities. It describes the different phases of fluid administration:
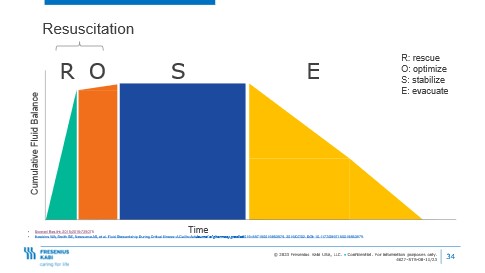
R – Rescue – rapid administration of fluids “as a life-saving measure” for fluid resuscitation
O – Optimization – careful adjustment of fluid input to optimize tissue perfusion. This might call for administration of fluids 250-300 mL at once, rather than one liter increments, said Dr. Hawkins
S – Stabilization – a phase that could take several days and involves maintaining organ perfusion
E – Evacuation – “This is where we are trying to remove fluid from the patient to achieve a net zero fluid balance” by pharmacological, mechanical or natural means.
In much of the literature the rescue and optimization phases are grouped together and described as ‘resuscitation’, he noted.
Clinicians also need to consider the ‘Four Rights’ framework for fluids, covering patient, drug, dose and route.2
Right patient – do they need IV fluids or a diuretic?
Right drug – which IV fluid to give, 0.9% sodium chloride or balanced crystalloids.
Right route – is it appropriate to give it IV or can it be given enterally?
Right dose – what is the appropriate dose, rate, duration and cumulative dose of fluid?
The ROSE model helps clinicians to identify the phase of fluid administration and this, in turn, determines the goal of fluid therapy. Once this is known, the Four Rights framework can be applied to determine the appropriate fluid treatment and construct a patient-centered plan.
Together these two models provide a basis for the practice of fluid stewardship. An algorithm has been developed to guide practitioners through the process.20 It could be used both for order verification and for prospective evaluation of the need for IV fluids, he said.
Dr. Hawkins and his colleagues analyzed more than 2500 patient care interventions and categorized them according to the Four Rights framework and the ROSE model.19 About 19% were related to fluid-stewardship and a large proportion of these were to do with ‘right patient’ and ‘right route’ and the stabilization phase of the ROSE model. “What that really boils down to is maintenance fluids” he said, noting that over 60% of hospitalized patients receive maintenance IV fluids and this accounts for 35% of their total fluid intake.21 “If you go back to your institution and you do nothing else, …. find patients that are on maintenance IV fluids and figure out if they are appropriate” he recommended.
Take-home message
References
- Van Regenmortel N, Verbrugge W, Roelant E et al. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med (2018) 44:409–417 https://doi.org/10.1007/s00134-018-5147-3
- Hawkins WA, Smith SE, Sikora Newsome A et al Fluid Stewardship During Critical Illness: A Call to Action. J Pharm Pract.2020 Dec; 33(6): 863–873. Published online 2019 Jun 30. doi: 1177/0897190019853979
- Boyd JH, Forbes J, Nakada TA et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011 Feb;39(2):259-6565. https://doi.org/10.1097/ccm.0b013e3181feeb15
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine 2006; 354: 2564-2575. 2006/05/23. DOI: 10.1056/NEJMoa062200.
- Vaara ST, Korhonen AM, Kaukonen KM, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Critical care (London, England) 2012; 16(5): R197. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682299/
- Neyra JA, Li X, Canepa-Escaro F, et al. Cumulative Fluid Balance and Mortality in Septic Patients with or without Acute Kidney Injury and Chronic Kidney Disease. Critical care medicine 2016; 44: 1891-1900. 2016/06/29. DOI: 10.1097/ccm.0000000000001835.
- Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomized, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016 42:1695-1705. https://doi.org/10.1007/s00134-016-4500-7
- Brotfain E, Koyfman L, Toledano R, et al. Positive fluid balance as a major predictor of clinical outcome of patients with sepsis/septic shock after ICU discharge. Am J Emerg Med 2016; 34: 2122-2126. 2016/08/25. DOI: 10.1016/j.ajem.2016.07.058.
- Shaw AD, Raghunathan K, Peyerl FW, et al. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive care medicine 2014; 40: 1897-1905. DOI: 10.1007/s00134-014-3505-3.
- Patel DM and Connor MJ, Jr. Intra-Abdominal Hypertension and Abdominal Compartment Syndrome: An Underappreciated Cause of Acute Kidney Injury. Advances in chronic kidney disease 2016; 23: 160-166. 2016/04/27. DOI: 10.1053/j.ackd.2016.03.002.
- Mitchell KH, Carlbom D, Caldwell E, et al. Volume Overload: Prevalence, Risk Factors, and Functional Outcome in Survivors of Septic Shock. Ann Am Thorac Soc 2015; 12: 1837-1844. DOI: 10.1513/AnnalsATS.201504-187OC.
- Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte (R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Annals of Surgery 256(1):p 18-24, July 2012. | DOI: 1097/SLA.0b013e318256be72
- Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 2012; 255: 821-829. DOI: 10.1097/SLA.0b013e31825074f5.
- Raghunathan K, Bonavia A, Nathanson BH, et al. Association between Initial Fluid Choice and Subsequent In-hospital Mortality during the Resuscitation of Adults with Septic Shock. Anesthesiology 2015; 123: 1385-1393. DOI: 10.1097/ALN.0000000000000861.
- Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama 2012; 308: 1566-1572. DOI: 10.1001/jama.2012.13356.
- Young P, Bailey M, Beasley R, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. Jama 2015; 314: 1701-1710. DOI: 10.1001/jama.2015.12334.
- Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Critical care medicine 2014; 42: 1585-1591. DOI: 10.1097/CCM.0000000000000305.
- Bissell BD and Mefford B. Pathophysiology of Volume Administration in Septic Shock and the Role of the Clinical Pharmacist. The Annals of pharmacotherapy 2020; 54: 388-396. 2019/11/07. DOI: 10.1177/1060028019887160.
- Semler MW, Self WH, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. The New England journal of medicine 2018; 378:;9 nejm.org March 1, 2018 https://www.nejm.org/doi/full/10.1056/NEJMoa1711584
- Hawkins WA, Butler SA, Poirier N et al From theory to bedside: Implementation of fluid stewardship in a medical ICU pharmacy practice. American Journal of Health-System Pharmacy, Volume 79, Issue 12, 15 June 2022, Pages 984–992, https://doi.org/10.1093/ajhp/zxab453
- Bihari S, Watts NR, et al. Maintenance fluid practices in intensive care units in Australia and New Zealand. Critical Care and Resuscitation; Volume 18 Number 2; June 2016 https://pubmed.ncbi.nlm.nih.gov/27242106/
 |
Based on a presentation given by Dr. W. Anthony Hawkins at a Fresenius Kabi satellite symposium held at the ASHP Midyear Clinical Meeting 3-7th December 2023. |
About W Anthony Hawkins
W. Anthony Hawkins, PharmD, BCCCP is a clinical associate professor at the University of Georgia College of Pharmacy and the Medical College of Georgia at Augusta University on the Southwest GA campus. He practices in an adult medical ICU at a large community teaching hospital and precepts pharmacy and medical students and pharmacy residents.
Dr. Hawkins obtained his PharmD from UGA and completed his critical care specialty residency at Emory University Hospital in Atlanta, GA. He is Board Certified in Critical Care Pharmacotherapy (BCCCP) and was named Fellow of American College of Critical Care Medicine in 2021. Dr. Hawkins is an appointed member of the Board of Directors for Georgia Society of Health System Pharmacists and the Research Advisory Council for the ASHP Foundation. He also serves ACCP, AACP, and SCCM in various capacities, such as mentor, committee chair, and others. His current research efforts focus on fluid stewardship practice and implementation and critical care education with the UGA Critical Care Collaborative (UGAC3), and he has published several peer reviewed papers in these focus areas.
<Back to Fresenius Kabi IV Fluid Stewardship Program homepage>





