Advertisment
Nivolumab study supports use of immune checkpoint inhibitors in advanced skin cancer
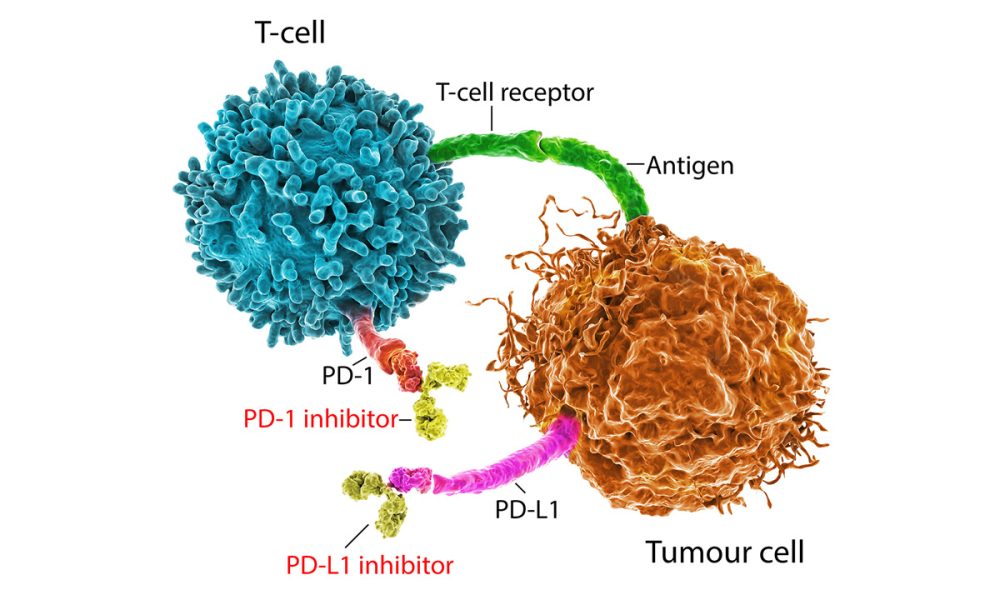
Researchers report that patients with advanced cutaneous squamous cell carcinoma, a skin cancer and one of the most frequent malignancies worldwide, benefit from treatment with the immune checkpoint inhibitor nivolumab. The findings were published on Oct. 24, 2022 in Cancer.
“This is the first study to investigate nivolumab in this patient population, and it provides further evidence supporting the use of immune checkpoint blockers as standard therapies in cutaneous squamous cell carcinoma,” said lead author Rodrigo R. Munhoz, MD, of the Hospital Sírio-Libanês, in Sao Paulo, Brazil.
While two immune checkpoint inhibitors, cemiplimab and pembrolizumab, have been approved by the US Food and Drug Administration for the disease, this new study is the first to report trial results for nivolumab.
The investigators conducted the phase 2 study to evaluate the safety and/or efficacy of nivolumab in treatment-naive patients with advanced cutaneous squamous cell carcinoma.
They administered nivolumab (3mg/kg) every 2weeks until disease progression, unacceptable toxicity or 12 months of treatment.
The primary end point was the best objective response rate.
Secondary end points included safety, progression-free survival (PFS), and overall survival (OS).
The researchers enrolled 24 subjects with a median age of 74 years (range, 48–93).
They reported that 14 of the subjects (58.3%) achieved a response to treatment.
Adverse events of any grade were reported in 87.5% of the subjects, with grade ≥3 occurring in six subjects (25%).
One patient discontinued nivolumab due to toxicities.
“With a median follow-up of 17.6 months, median duration of response has not been reached, and the estimated median PFS [progression-free survival] and OS [overall survival] were 12.7 and 20.7 months, respectively,” the authors said.
The authors concluded, “Nivolumab resulted in robust antitumor activity, sustained responses, and good tolerability in systemic treatment-naive patients with aCSCC [advanced cutaneous squamous cell carcinoma]. These data provide further evidence to support the use of ICI [immune checkpoint inhibitor] as the standard treatment of aCSCC.





