Advertisment
TGA in Australia has granted provisional registration for Spikevax COVID 19 vaccine for children aged 6-11 years old – Moderna
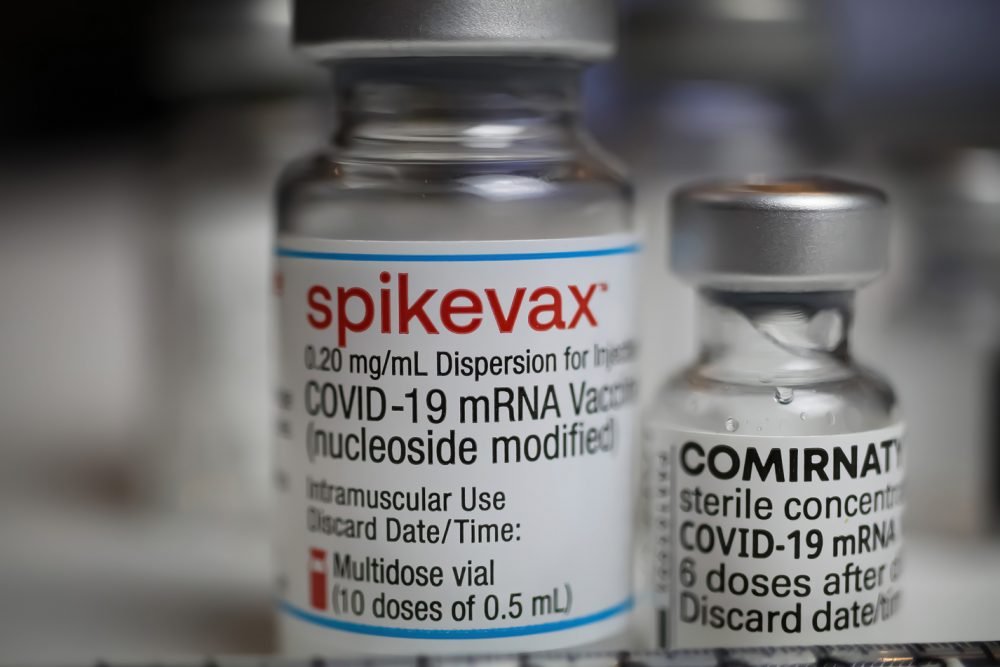
Moderna, Inc., announced that the Therapeutic Goods Administration (TGA) in Australia has granted provisional registration for the use of Moderna’s mRNA COVID-19 vaccine, Spikevax, in a 50 µg dose, two-dose series, for active immunization to prevent COVID-19 caused by SARS-CoV-2 in children aged 6-11 years.
The TGA authorization for the use of the COVID-19 vaccine in children 6-11 years old in Australia is an important milestone for Moderna as it is the first regulatory authorization for the use of Spikevax in this age group.
Professor Robert Booy from the Immunisation Coalition commented, “I welcome this decision by the TGA and look forward to the uptake of vaccination in children increasing even more to provide protection of children and maximize school attendance.”