Advertisment
Phase III MIRROR trial of Krystexxa meets primary endpoint in chronic gout – Horizon Therapeutics
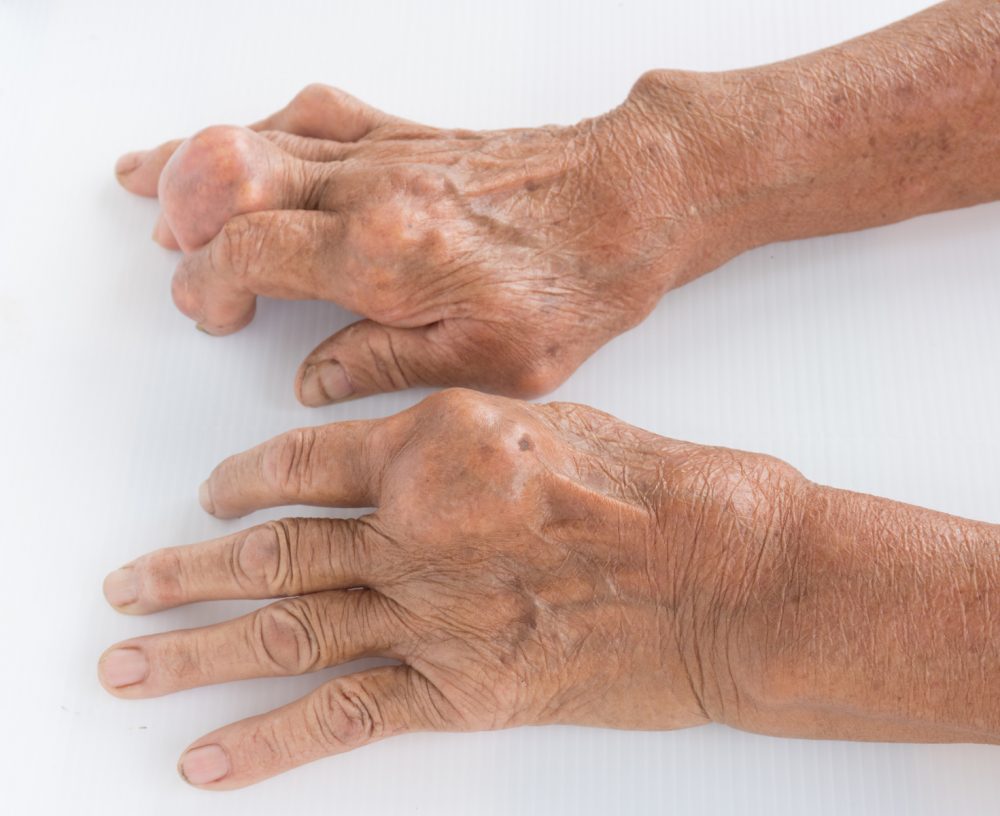
Horizon Therapeutics announced topline results of the phase III MIRROR trial met the primary endpoint, showing a significant increase in efficacy using Krystexxa (pegloticase injection) with the immunomodulator methotrexate as compared to the response rate of Krystexxa with placebo for people with chronic gout refractory to conventional therapies – also known as uncontrolled gout. Results were from the Methotrexate to Increase Response Rates in Patients with Uncontrolled Gout Receiving Krystexxa trial [MIRROR randomized controlled trial (RCT)].
Results show that 71% (71 of 100) of patients who were randomized to receive Krystexxa with methotrexate compared to 40% (21 of 52) of patients who were randomized to receive Krystexxa with placebo achieved the primary endpoint (p<0.001). No new safety concerns were identified. These results are aligned with published literature and the prior original pivotal clinical trials of Krystexxa monotherapy which showed 42% (36 of 85) of dosed patients had a complete sUA response and met the primary endpoint of maintaining sUA <6 mg/dL at least 80% of the time in Months 3 and 6. Data from the trial are expected to be presented at an upcoming medical congress. Horizon plans to submit a Supplemental Biologics License Application (sBLA) to the FDA in the first quarter of 2022.





