Advertisment
Ivermectin systematic review confirms efficacy in covid-19

Article written by Christine Clark.
The long-awaited systematic review of ivermectin trials from Dr Tess Lawrie’s team at the Evidence-based Medicine Consultancy has now been published in the peer-reviewed American Journal of Therapeutics.1 This review confirms that ivermectin reduces the risk of dying from covid-19 and is effective in preventing infection.
The authors conclude:
“Given the evidence of efficacy, safety, low cost, and current death rates, ivermectin is likely to have an impact on health and economic outcomes of the pandemic across many countries. ……. Ivermectin is likely to be an equitable, acceptable, and feasible global intervention against COVID-19.
Health professionals should strongly consider its use, in both treatment and prophylaxis.”
True to form, the authors have conducted a rigorous and exacting review of the evidence to determine the efficacy of ivermectin treatment in reducing mortality, in secondary outcomes and in prophylaxis for people with or at high risk of covid-19 infection.
After sifting through numerous trial reports, 24 trials, involving 3406 participants were included in the review.
Treatment of covid-19
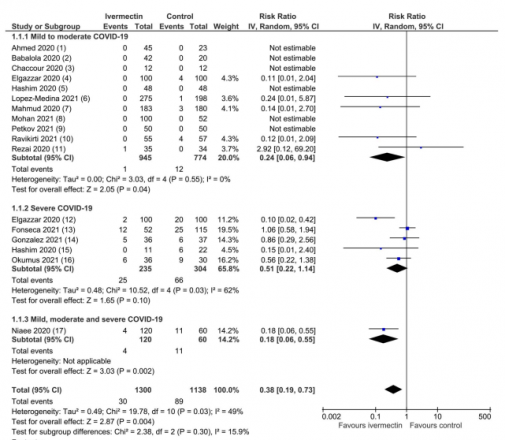
A meta-analysis of 15 trials, including 2438 participants, found that ivermectin reduced the risk of death by an average of 62% (average risk ratio 0.38, 95% CI 0.19-0.73) compared with no ivermectin, in hospitalised patients. In absolute terms, this corresponds to a risk of death of 2.3% with ivermectin versus 7.8% with no ivermectin. This was graded as ‘moderate-certainty’ evidence using the GRADE criteria. It is perhaps worth remembering that there are only four certainty levels and ‘moderate’ is only one step below the top level – ‘high’. ‘Moderate-certainty’ means that the authors believe that the true effect is probably close to the estimated effect. To put this in context – remdesivir was recommended on the basis of two trials with 1300 participants with ‘low-certainty’ evidence.
Prophylaxis for covid-19
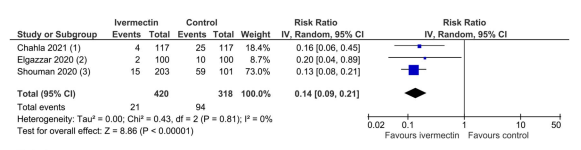
Three trials involving 738 participants were included in the meta-analysis for prophylaxis. This showed that ivermectin reduces the risk of infection by an average of 86% (79% – 91%; average risk ratio 0.14, 95% CI 0.09 – 0.21). In absolute terms, 5% of participants contracted covid with ivermectin versus 29% with no ivermectin. This was graded as ‘low-certainty’ evidence because of study design limitations and the small number of trials.
Other points to note
RCTs only: This review was restricted to randomised controlled trials (RCTs), including trials published on preprint sites which have usually not undergone peer review. This was justified on the grounds that the studies were peer-reviewed by the authors during the review process and original authors were contacted for additional information if necessary.
Rigorous statistics: This review has applied rigorous statistical techniques, including trial sequential analysis (TSA) to ensure that the analysis is robust. It is of interest that the TSA confirms that the required ‘information size’ has probably already been met. The ‘information size’ is the total number of trial participants needed to have a realistic chance of determining the true effect size.
Ivermectin is a cheap drug: A recent estimate of cost from Bangladesh reports a cost of US$0.60-1.80 for a five-day course of ivermectin.
What next?
It has been announced today (23rd June 2021) that ivermectin is to be investigated as a possible treatment for covid-19 in the Oxford PRINCIPLE trial. An explanatory article on the PRINCIPLE website acknowledges that “ivermectin is a safe, broad spectrum antiparasitic drug which is in wide use globally to treat parasitic infections”
Professor Chris Butler, from the University of Oxford’s Nuffield Department of Primary Care Health Sciences, Joint Chief Investigator of the PRINCIPLE trial, said, “Ivermectin is readily available globally, has been in wide use for many other infectious conditions so it’s a well-known medicine with a good safety profile, and because of the early promising results in some studies it is already being widely used to treat COVID-19 in several countries. By including ivermectin in a large-scale trial like PRINCIPLE, we hope to generate robust evidence to determine how effective the treatment is against COVID-19, and whether there are benefits or harms associated with its use.”
So far no protocol has been published. It is to be hoped that the trial will be started without further delays.
We remind viewers that real world experience of the use of ivermectin has been much more than merely ‘promising’. Interviews with Dr Suryakant in Lucknow, India, and Dr Jackie Stone in Harare, Zimbabwe provide personal testimony from physicians involved in direct patient care. Dr David Scheim has described the dramatic reductions in excess deaths after mass treatment (with ivermectin) of the population in Peru, and Dr Tess Lawrie herself described her first meta-analysis of ivermectin studies in January 2021. It is salutary to remember that at that time she said, “In this instance, where there’s such a massive effect and it is so consistent, doing a big trial is not going to come up with something different in the opposite direction”.
Reference
Bryant A, LawrieTA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, Tham TC. Ivermectin for Prevention and Treatment of COVID-19 Infection. American Journal of Therapeutics: June 17, 2021 – Volume Publish Ahead of Print – Issue – doi: 10.1097/MJT.0000000000001402





